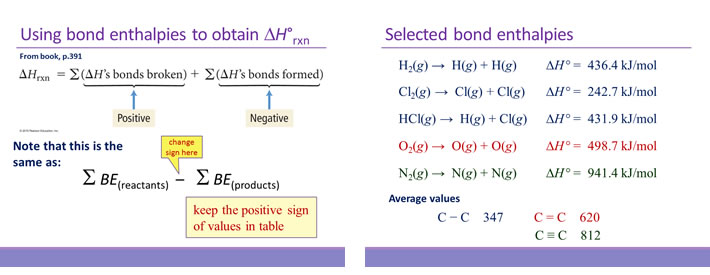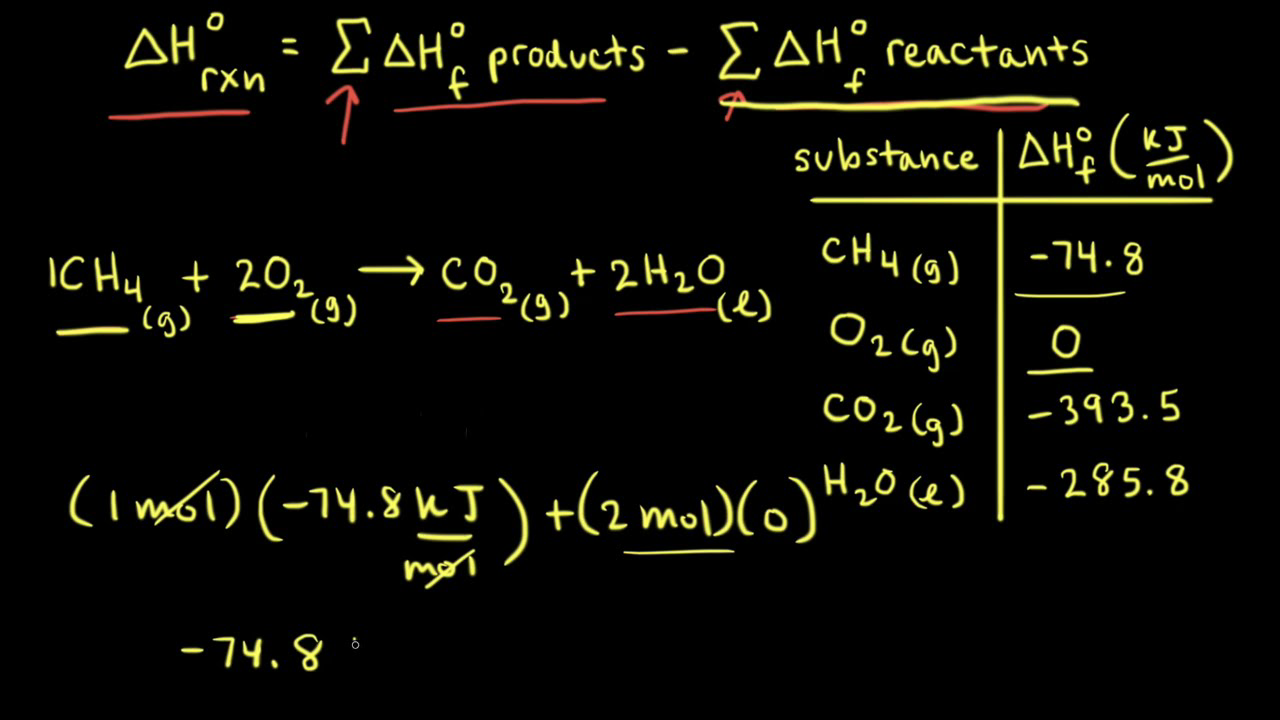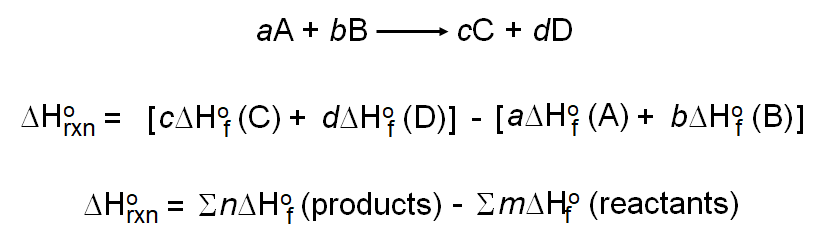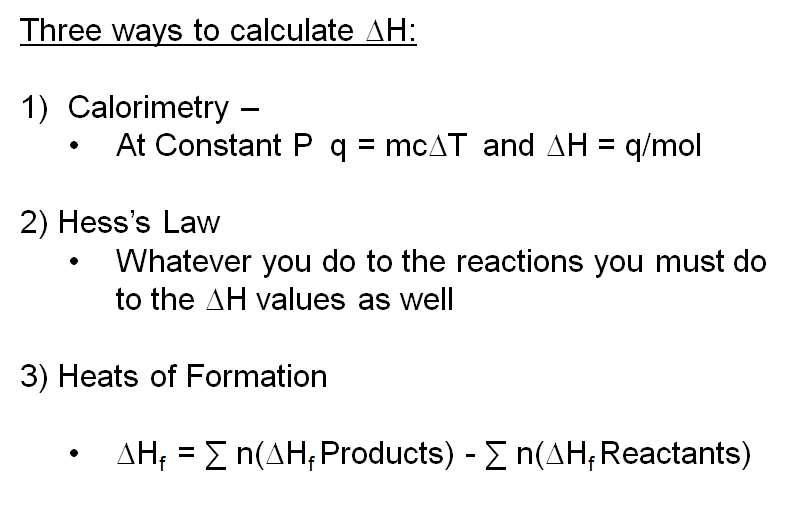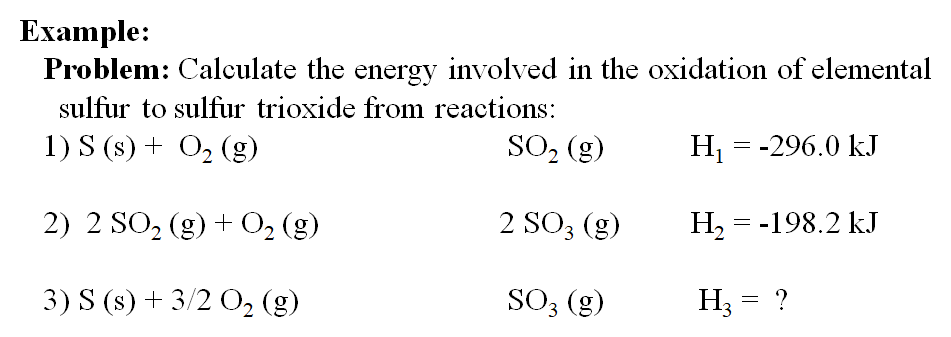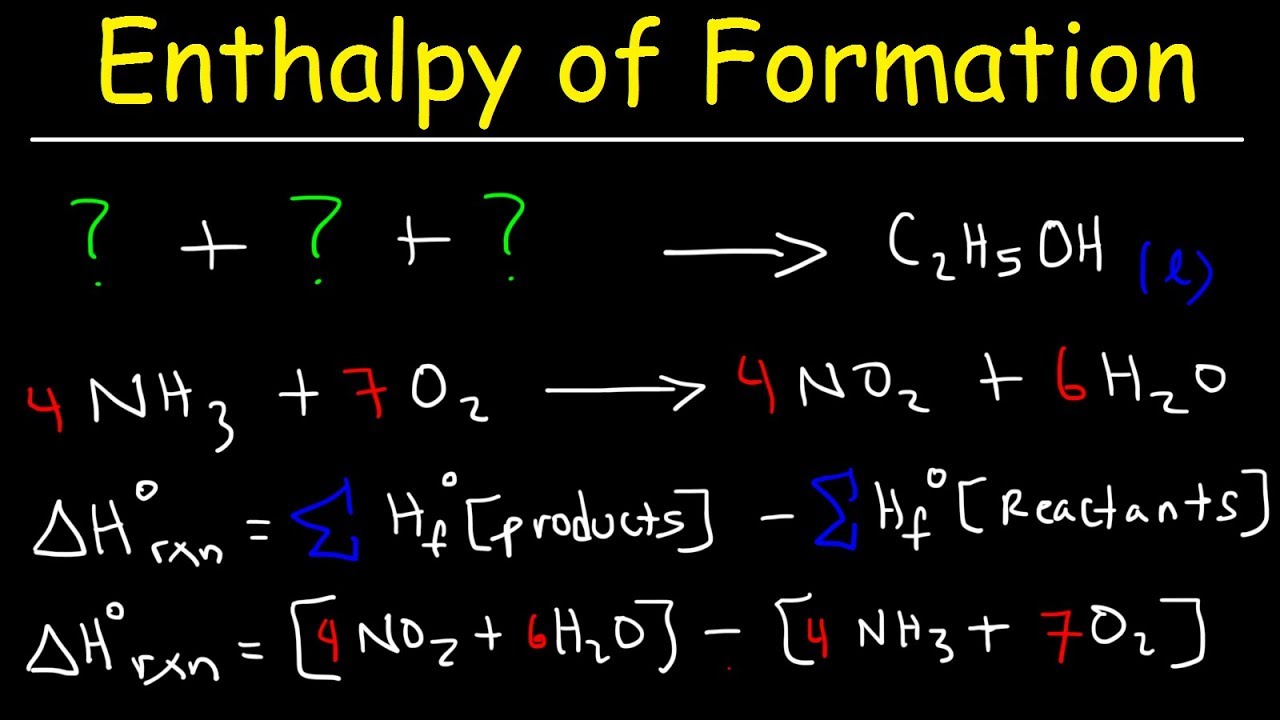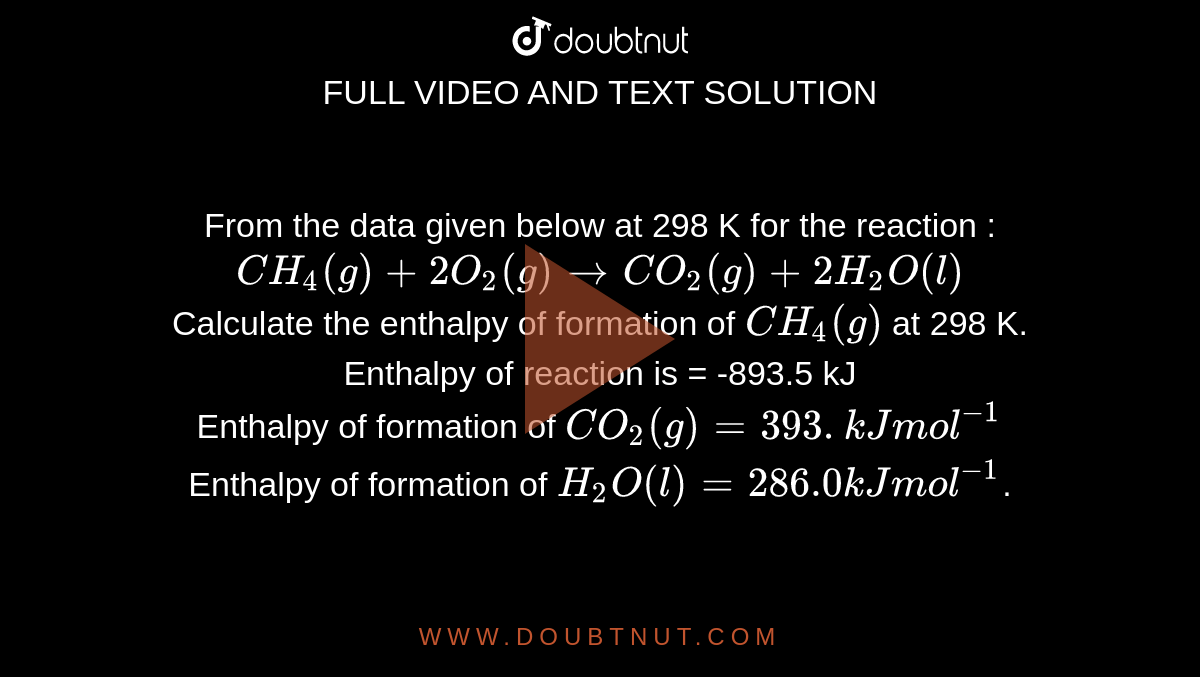
Calculate the enthalpy change for the following reaction: CH(4)(g)+2O(2)(g)toCO(2)(g)+2H(2)O(l) given, enthalpies of formation of CH(4),CO(2) and H(2)O are -74.8kJ mol^(-1),-393.5kJ" "mol^(-1) and -286.2kJ" "ml^(-1) respectively.

SOLVED: Question 3 Calculate the Enthalpy of the Photosynthesis Reaction 0/1 points Balance the reaction shown below that occurs during photosynthesis and use bond energies to estimate the enthalpy change for the

SOLVED: Hess' Formula Using the A,H" values in your Data Booklet, calculate the heat of reaction (enthalpy change) for each of the following and draw the potential energy (Ep) diagram: (4 marks

thermodynamics - Calculating Enthalpy of formation versus Calculating Enthalpy of a reaction not occurring at standard conditions - Chemistry Stack Exchange