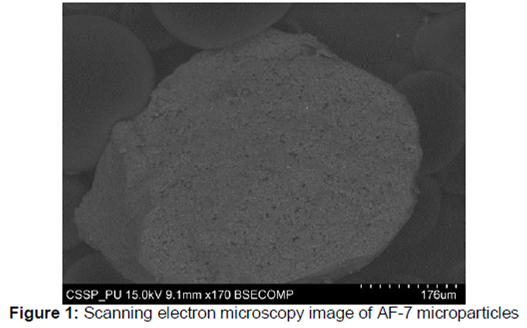
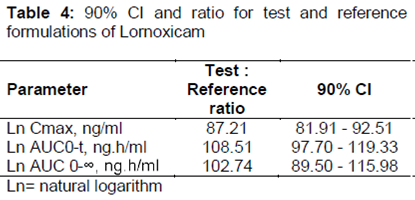
PDF) MODUL BIOETICA CERCETARII STIINTIFICE PE SUBIECTUL UMAN SI ETICA PUBLICARII STIINTIFICE CICLUL I -PROGRAMUL DE PREGATIRE UNIVERSITARA AVANSATA. MODUL OBLIGATORIU | Rares Munteanu - Academia.edu

PDF) A Model Data Management Plan Standard Operating Procedure: Results From the DIA Clinical Data Management Community, Committee on Clinical Data Management Plan

Efficacy and Speed of Onset of Pain Relief of Fast-Dissolving Paracetamol on Postsurgical Dental Pain: Two Randomized, Single-Dose, Double-Blind, Placebo-Controlled Clinical Studies - ScienceDirect
ETHIK-KOMMISSION DER MEDIZINISCHEN UNIVERSITÄT WIEN E-Mail: ethik-kom@ meduniwien.ac.at ethikkommission.meduniwien.ac.at
FDA/MHRA Good Clinical Practice Workshop Day 2- October 24, 2018 Session 2 - Case Studies on BE/GCP Date Management Case Study (